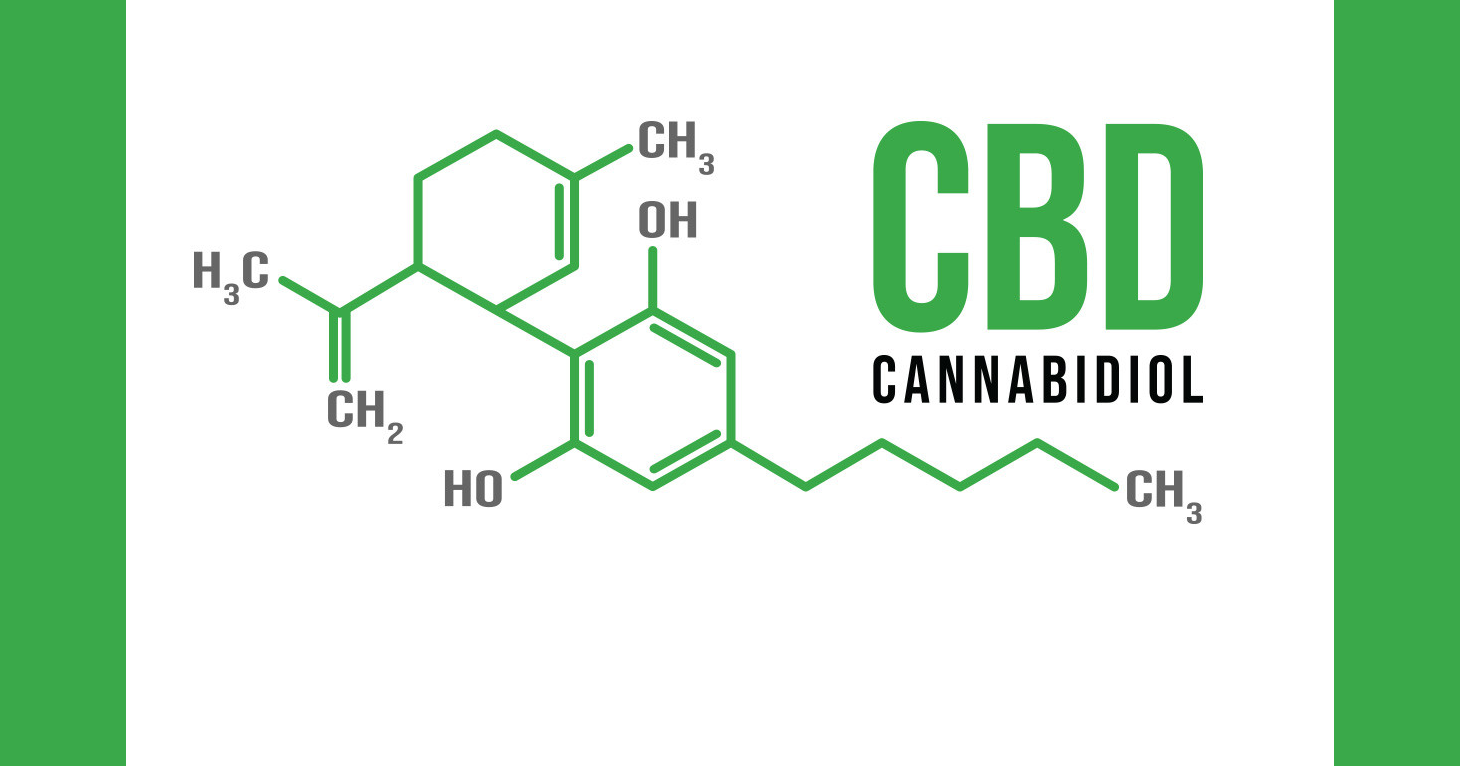
SeraRelief CBD products
Be wary of “free trials” for CBD gummies and oils advertised on the internet.
 When it comes to the health benefits of CBD, it is often the case that the science says one thing and the marketing says another.
When it comes to the health benefits of CBD, it is often the case that the science says one thing and the marketing says another.
Take cancer. According to the National Cancer Institute (NCI), cannabinoids in the cannabis plant like non-high-inducing CBD may help treat the side effects of cancer. But clinical trials that study cannabis for cancer treatment are limited and there are no such ongoing studies in a vast database of more than 30 million citations maintained by the National Institutes of Health, NCI says.
You can’t rush the science. Yet many marketers of CBD products including, until recently, NuLife, tout their CBD-infused capsules, oils and creams as a proven treatment for cancer, among other serious diseases. It seems it’s not until they are faced with the threat of legal action that they begin to tighten up their marketing.
Such was the fate of NuLife, which, after receiving an FTC warning letter last month, made extensive edits to a medical applications page on its website that had recommended CBD for everything from cancer to Alzheimer’s disease to autism. These claims are gone.
In addition, other language has been toned down. For example, where NuLife used to refer to the “health applications of CBD,” the company now cites the “possible benefits of CBD.” However, in another section of the website, NuLife still carries three product reviews that claim the company’s creams and oils help with arthritis and high blood pressure, despite the FTC flagging the reviews in its letter, which warned:
It’s not enough that an endorsement represents the consumer’s honest opinion or experience. Reasonable consumers may interpret an endorsement claiming a health benefit from the use of a product as representing that the product is likely to be effective in achieving that benefit. Under FTC law, an advertiser must possess and rely on competent and reliable scientific evidence to support health claims, both express and implied, made through the use of endorsements.
The FTC also sent warning letters to the marketers of Ocanna and Magic Green Oil Drops CBD products. TINA.org obtained the letters through a Freedom of Information Act request.
Read more about our coverage on CBD here.
Be wary of “free trials” for CBD gummies and oils advertised on the internet.
CBD “super store” brochure is chock full of unapproved disease-treatment claims.
Disease-treatment claims vanish in wake of FTC warning letter.