Snow Teeth Whitening
TINA.org investigated Snow Teeth Whitening, an Arizona-based dental cosmetics company, and found that it was improperly using an FDA logo in its marketing materials, including on its product packaging, website, and social media platforms, in violation of the FDA’s Logo Policy, as well as falsely claiming that its teeth whitening products were FDA-approved.
TINA.org also investigated Snow Teeth Whitening as part of its larger investigation into deceptive social media influencer marketing. To learn more, click here.

Highlights
- Filed complaint with FDA
- Company removed FDA logo and "FDA-approved" claims from its packaging and marketing
Timeline
2019
August 8
TINA.org sends a follow-up letter to the FDA regarding Snow Teeth Whitening’s continuing use of the FDA logo in certain marketing materials, including webpages and social media platforms.
July 22
Snow Teeth Whitening’s product packaging (as shown on its website and social media pages) no longer bears the FDA logo. (See this before and after.)
July 17
Snow Teeth Whitening no longer claims on its website that its teeth whitening kit is “FDA approved.” (See this before and after.)
July 10
TINA.org sends a complaint to the FDA regarding Snow Teeth Whitening’s improper use of an FDA logo.
2018
June 15
As shown in TINA.org’s Social Media Influencer investigation, Snow Teeth Whitening is promoted by social media influencer Dorothy Wang, who failed to include adequate disclosures of her material connection to the brand in her posts.
Evidence
Featured

What’s the FDA’s Logo Doing on Snow Teeth Whitening’s Packaging?
Agency prohibits its logo from appearing on private sector materials. Snow says it has connections.
The Latest
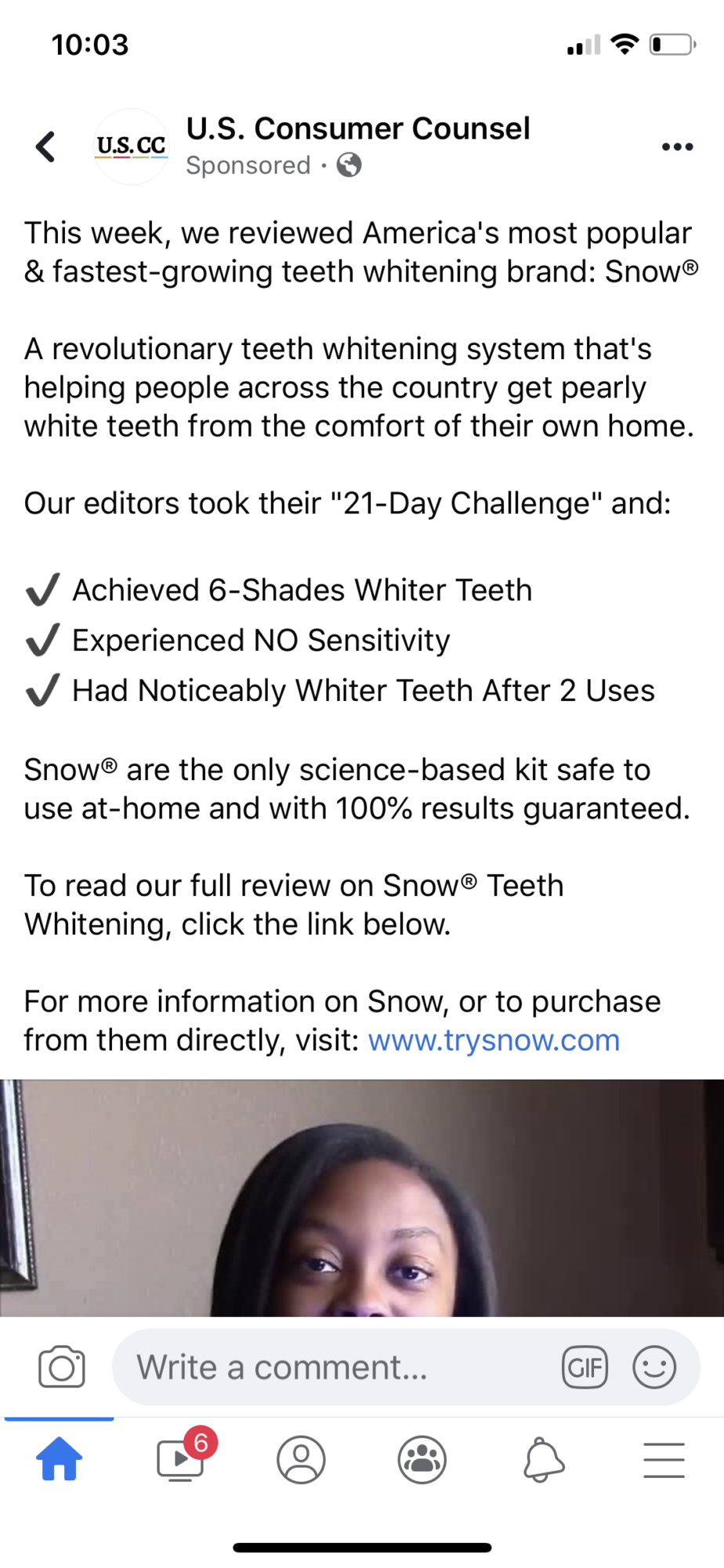
U.S. Consumer Counsel aka U.S. Consumer Times
Sketchy affiliate website promoting Snow Teeth Whitening comes down in wake of TINA.org inquiry.

5 Ad Trends to Be Wary of in 2020
CGI influencers are here.
Class-Action Tracker

Snow Teeth Whitening
Allegations: Falsely marketing Snow Teeth Whitening